Introduction
Bioprinting can be defined as the use of computer-aided transfer processes for patterning and assembling living and non-living materials with a prescribed 2D or 3D organization in order to produce bio-engineered structures serving in regenerative medicine, pharmacokinetic and basic cell biology studies. (International Conference about Bioprinting and Bio Manufacturing – Bordeaux 2009). Bioprinting is based on the principle of the three “B”: Bioink, Biopaper, Bioprinter (see Dababneh2014 for a general overview about the topic).
Goal
Our aim is to develop a versatile bio-printing platform by a modular approach inspired by the Bioplotter realized by Lee2009. In particular, we foresee 2 steps:
- Develop a smart cartridge (micro-electrovalve) able to dispense bio-ink keeping at the same time cellular function on other biological requirements;
- Integrate the micro-electrovalve with a 3D printer platform to build 3D structures.
Such a development is performed by the key collaborations among three partners: CompMech group (UniPV), Dept. of Molecular Medicine of the University of Pavia, and F-Lab Company.
A first prototype of the smart-cartridge is depicted in Figure 1.
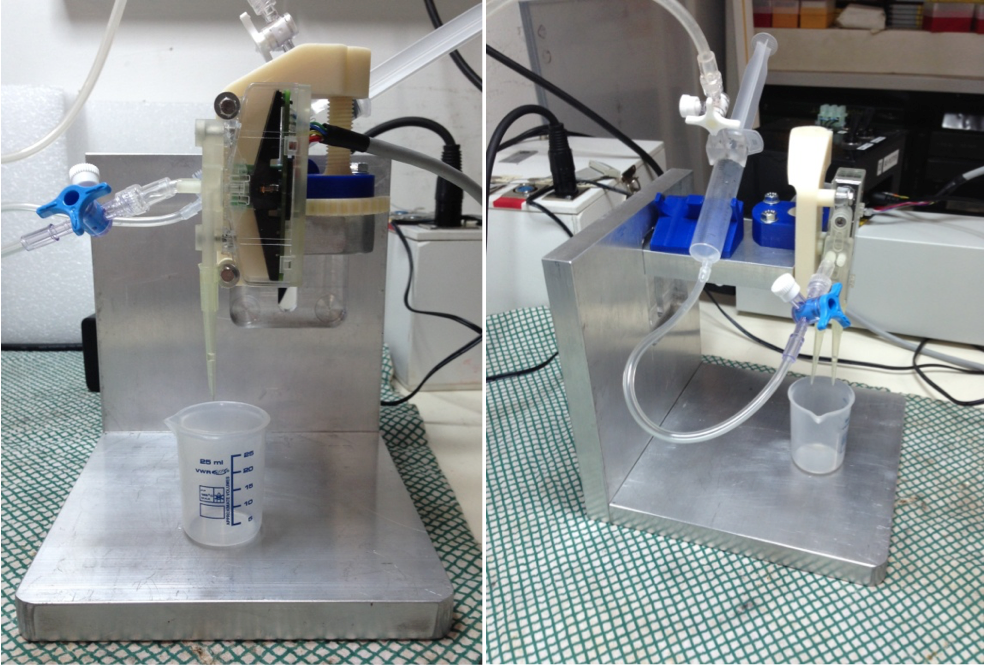
Fig. 1: Details of the first prototype of the smart dispenser based on DFD valve by F-Lab.
Video: Droplet dispensing by micro-electro-valve
Preliminary Tests
Preliminary tests have been already performed to assess the capability of DFD valve to control micro-flows as shown by the plot in Figure 2, where the characteristic curves of each channel of the valve are depicted.
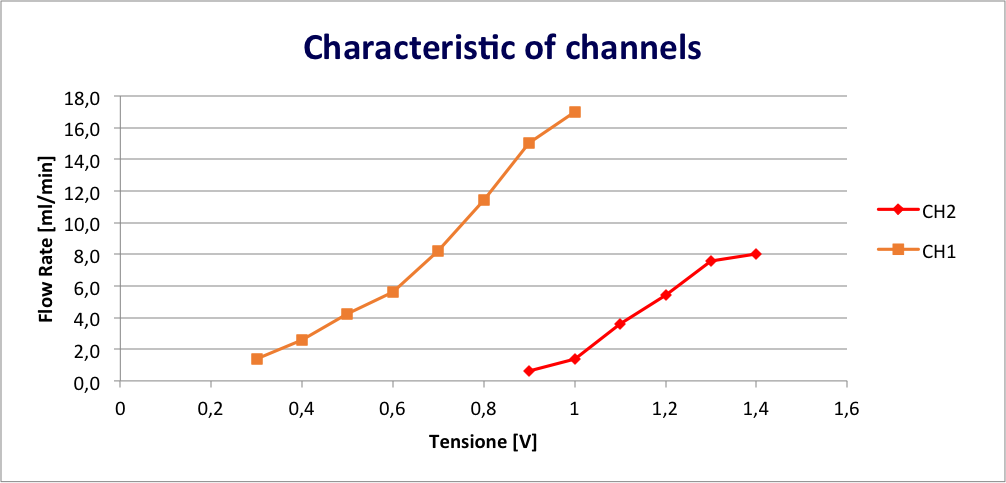
Fig. 2: Plot of the flow rate [ml/min] as a function of the applied voltage [V]
Liquid solutions with different viscosities (Glycerol solutions reported in Table 1) has been dispensed as well, pawing the way to the use of silk-based hydrogel.
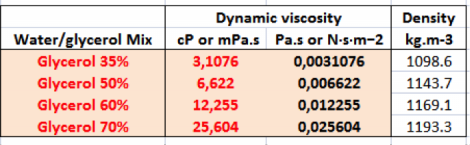
Table 1: Technical data relating to water and glycerol solutions: red highlights the solutions tested in the laboratory.
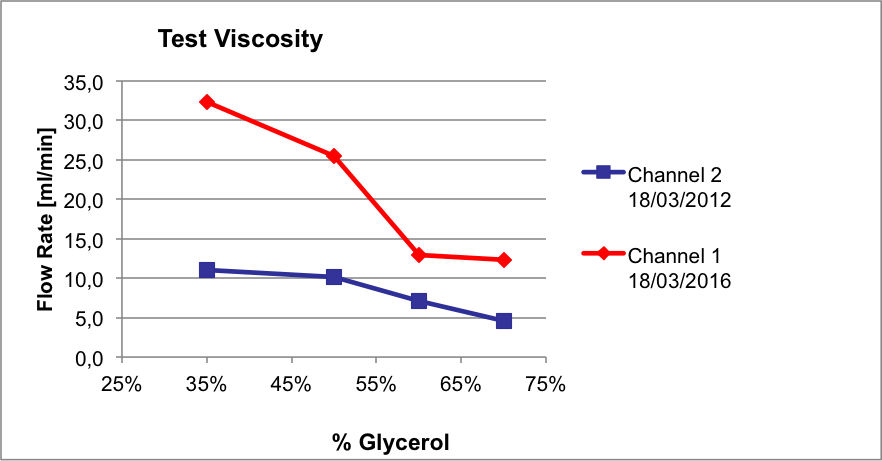
Fig. 3: Plot of the flow rate [ml/min] as a function of the % Glycerol
The results from the viscosity tests are particularly encouraging, as we were able to extrude materials with a dynamic viscous up to 25 cP. There are, therefore, good possibilities to carry out tests with silk-based solutions which present a viscosity of 13 times higher than water.
References
- Lee, Wonhye, et al. “Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication.” Biomaterials 30.8 (2009): 1587-1595.
- Dababneh, Amer B., and Ibrahim T. Ozbolat. “Bioprinting technology: a current state-of-the-art review.” Journal of Manufacturing Science and Engineering 136.6 (2014): 061016.
Collaborations
- Dept. of Molecular Medicine of the University of Pavia, Prof. Alessandra Balduini’s lab
- Dolphin Fluidics/F-Lab
Acknowledgements
- Cinzia Ferrari, University of Pavia, Dept. of Clinical and Surgical Sciences Experimental Surgery Laboratory